Knauss legislative fellowships in Congress help build careers — and they're fun and educational. See our video and fact sheet for details.
What Goes on Beneath an Oyster Cage?
Oysters are amazing filter feeders that slurp up algae and improve water quality in the Chesapeake Bay. After they digest algae, they expel their waste onto the Chesapeake Bay floor. On the Bay floor, bacteria love oyster waste, and naturally break down these particles. However, when too many oysters live in a location that experiences stagnant water; the buildup of oyster waste can be too much. Instead of getting carried away by flowing water, the waste builds up underneath the cages.
This oyster waste is a food source for the wide variety of bacteria that live along the water bottom or are buried in the sediment below. The chemical processes these bacteria use to break down oyster waste can be compared to how humans break down food for energy: There are quicker, easier processes, and longer, more complicated processes.
The simplest process takes place on the Bay floor, where bacteria use oxygen as a fuel source to help them break down oyster waste. But eager bacteria consume the oxygen near them quickly. This is similar to how humans use the fuel from carbohydrates like chips or crackers—they are a quick, easy energy source for us to digest, but we use up the energy quickly. Bacteria on the Bay floor try to keep up, but at times, there is more oyster waste than there is oxygen available for the bacteria who use oxygen to break down the oyster waste.
That's when the bacteria in the deeper layers of the sediment have an opportunity to break down the oyster waste. These bacteria use more complicated pathways to break down the oyster waste, since less oxygen is available to them. These more complicated processes take more time. A comparable, more complicated pathway in humans is how we use proteins like beef for fuel. Those energy sources take us more time to digest, so our body uses energy from carbohydrates first. Once the carbohydrates are used up, our body turns to more complicated pathways.
Likewise, the digestion process for oyster waste in the deeper layers of sediment is a more complicated process. Since there’s no oxygen to use as an easy fuel source, these bacteria can consume sulfate to break down oyster waste. This reaction is natural, but unfortunately produces a toxin called sulfide—the same toxin that gives rotten eggs their smell. When oyster waste accumulates in high levels, bacteria begin to break down the waste using sulfate. Consequently, the sulfide can build up in sediments underneath an oyster cage and harm the worms and plants that live around the oyster cage.
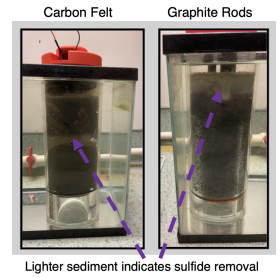
What am I doing to reduce the sulfide buildup? I am using carbon felt and graphite rods to attract bacteria that can use up the toxic sulfide under the oyster cages. These specialized bacteria that consume sulfide already live in the Chesapeake Bay. The sulfide-consuming bacteria can transfer electrons outside of their body, allowing them to connect to the graphite rods or carbon felt. The carbon felt and graphite rods give electrons to the bacteria, allowing them to remove toxic sulfide quickly.
Has this strategy worked? It is a work in progress. Last year, I maintained my own small-scale oyster farm of 16 cages at Horn Point Laboratory. I tested carbon felt and graphite rod prototypes and placed them underneath oyster bottom cages. The carbon felt prototype was destroyed by tidal forces, especially during Tropical Storm Ophelia. That setback led us to minimal sulfide removal data, but we plan to repeat the experiment, so stay tuned for updates!
Top left photo: Oysters and sea squirts in a mesh bag. Photo: Clara Fuchsman
See all posts to the Fellowship Experiences blog