Hunting for Hemocytes
Instructional Level: High School
Lesson Plan: Eastern Oyster Education
Driving Question(s):
How do environmental, ecosystem and anthrogenic impacts influence or interact with the anatomical structures and functions of the eastern oyster and related conservation and restoration efforts of this keystone species?
Exploration
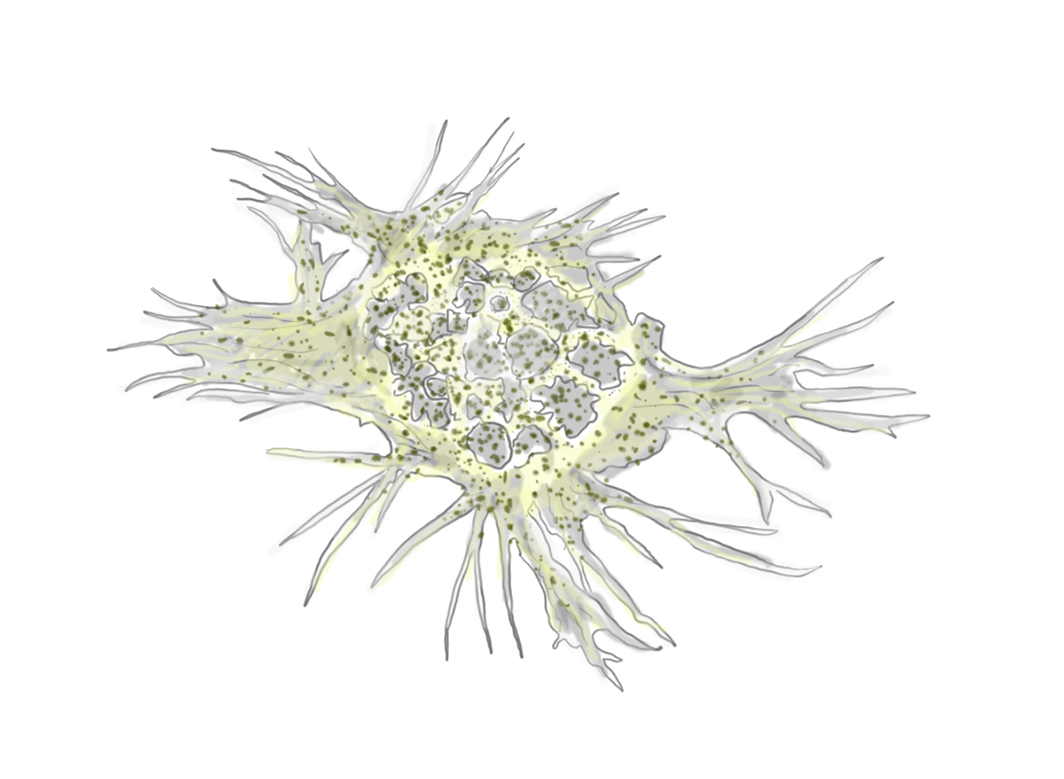
Hemocyte - sketch from life. J.A. Frederick
Mollusks, like many other invertebrates, have an open circulatory system that does not confine hemolymph to traditional vessels like veins, arteries, and capillaries. Instead, an open circulatory system will circulate hemolymph through a number of cavities and sinuses in various parts of the organism. In an oyster, the hemolymph is circulated in this way and can be readily found in the pericardial cavity that contains the heart. Within the hemolymph of the oyster there are three basic types of hemocytes that perform a wide variety of functions from defense to nutrient transport.
The procedure for harvesting hemocytes were developed in collaboration with Dr. Gerardo Vasta of the Institute of Marine and Environmental Technology in Baltimore along with Dr. Eric Schott, Dr. Jose A.F. Robledo, and Wolf Pecher in 1998. Video clips of hemocytes were produced in collaboration with Shannon Hood at UMCES Horn Point Laboratory. Their assistance has been an invaluable part of the translation of these practical techniques. The procedure is simple and requires only a few pieces of equipment. Viewing hemocytes is best accomplished with a progression of magnification from 10X to 20X to 40X. Lower magnification will allow you to see them but not in great detail. Follow the procedure and you will be able to see these cells live on a glass slide.
A readily found hemocyte, known as a granulocyte, can make a very impressive showing under 40X magnification to 60X magnification. These hemocytes appear to be filled with small "grains" and have long pseudopodia that extend from the outer surface of the cell. The pseudopodia are used for mobility and the capture of foreign bodies and disease causing organisms like dermo, Perkinsus marinus.
This activity combines a hands-on exploration with an online resource. Teachers can walk through the activity with the entire class or students can work independently.
Objectives
Students will:
- Observe and describe the immune system of the eastern oyster and identify key cells involved in the process.
- Observe and describe/illustrate the different types of oyster hemocytes.
- Connect the role of oyster hemocytes to the health of the Chesapeake Bay.
Lesson Materials
For Lab:
- Oyster knife
- Sturdy gloves
- Artificial saltwater (10-15ppt)
- Computer access
Per Lab Group:
- Live oysters
- Shallow glass or plastic dishes (min 2” depth)
- Compound light microscope with 10X, 20X and 40X objectives
- Glass slides and glass cover slips
- Probe
- Glass capillary pipette, micropipette or glass eyedropper
Procedures
Preparation
- For optimum results, oysters should be held in a saltwater tank for 24 hours prior to the lab.
- Shuck 1 oyster per lab group and 1 oyster for demonstration (for safety purposes it is not advised to have students shuck their own oysters). Use the hinge method to shuck the oyster to ensure that its internal anatomy stays intact. In this method, you will be removing the right valve of the oysters – be sure to leave the oyster attached to its left valve.
- Place oyster (in its left valve) in a dish without water.
Explore
- Give each student group their own shucked oyster/dish combination.
- As a class or independently, have students access and complete the activity Hemocyte Lab.
Explain
- Working in groups, have students compare and contrast the oyster hemocytes with their own white blood cells and how the immune system of the oyster is different from that of a mammal.
Evaluate
- Have students discuss, write or illustrate (drawing or video) how an oyster’s immune system protects the oyster from disease and the potential impact on the oyster reef habitat.
Standards
Next Generation Science Standards
Performance Expectation: HS-LS1-2
SEP: Developing and Using Models
- Modeling in 9–12 builds on K–8 experiences and progresses to using, synthesizing, and developing models to predict and show relationships among variables between systems and their components in the natural and designed worlds.
- Develop and use a model based on evidence to illustrate the relationships between systems or between components of a system. (HS-LS1-2)
- DCI: LS1.A: Structure and Function
- Multicellular organisms have a hierarchical structural organization, in which any one system is made up of numerous parts and is itself a component of the next level. (HS-LS1-2)
- CCC: Systems and System Models
- Models (e.g., physical, mathematical, computer models) can be used to simulate systems and interactions— including energy, matter, and information flows—within and between systems at different scales. (HS-LS1-2)
Performance Expectations HS-LS2-6
- SEP: Engaging in Argument from Evidence
- Engaging in argument from evidence in 9–12 builds on K–8 experiences and progresses to using appropriate and sufficient evidence and scientific reasoning to defend and critique claims and explanations about the natural and designed world(s). Arguments may also come from current scientific or historical episodes in science.
- Evaluate the claims, evidence, and reasoning behind currently accepted explanations or solutions to determine the merits of arguments. (HS-LS2-6)
- Engaging in argument from evidence in 9–12 builds on K–8 experiences and progresses to using appropriate and sufficient evidence and scientific reasoning to defend and critique claims and explanations about the natural and designed world(s). Arguments may also come from current scientific or historical episodes in science.
- DCI: Ecosystem Dynamics, Functioning, and Resilience
- A complex set of interactions within an ecosystem can keep its numbers and types of organisms relatively constant over long periods of time under stable conditions. If a modest biological or physical disturbance to an ecosystem occurs, it may return to its more or less original status (i.e., the ecosystem is resilient), as opposed to becoming a very different ecosystem. Extreme fluctuations in conditions or the size of any population, however, can challenge the functioning of ecosystems in terms of resources and habitat availability. (HS-LS2-6)
- CCC: Stability and Change
- Much of science deals with constructing explanations of how things change and how they remain stable. (HS-LS2-6)
Performance Expectation: HS-LS4-5
- SEP: Engaging in Argument from Evidence
- Engaging in argument from evidence in 9-12 builds on K-8 experiences and progresses to using appropriate and sufficient evidence and scientific reasoning to defend and critique claims and explanations about the natural and designed world(s).
- Arguments may also come from current or historical episodes in science.
- Evaluate the evidence behind currently accepted explanations or solutions to determine the merits of arguments. (HS-LS4-5)
- Engaging in argument from evidence in 9-12 builds on K-8 experiences and progresses to using appropriate and sufficient evidence and scientific reasoning to defend and critique claims and explanations about the natural and designed world(s).
- DCI: LS4.C: Adaptation
- Changes in the physical environment, whether naturally occurring or human induced, have thus contributed to the expansion of some species, the emergence of new distinct species as populations diverge under different conditions, and the decline–and sometimes the extinction–of some species. (HS-LS4-5)
- CCC: Cause and Effect
- Empirical evidence is required to differentiate between cause and correlation and make claims about specific causes and effects. (HS-LS4-5)
Maryland E-Lit Standards
- (2) Human Dependence on Earth Systems and Natural Resources. Environmentally literate students construct and apply understanding of how Earth’s systems and natural resources support human existence.
- (3) Environmental Impact of Human Activity. Environmentally literate students construct and apply understanding of the environmental impact of human activities on Earth’s systems and resources.
References
Hinge method of shucking an oyster
https://www.researchgate.net/publication/289639130_Structure_and_Functions_of_Oyster_Hemocytes
https://www.youtube.com/watch?v=PNZd178UYY0
Assessing the Presence and Virulence of Dermo Disease in the Environment Using a PCR-Based Diagnostic Assay for the Extrachromosomal Plastid Genome of Perkinsus marinus. 1996-1997. Gerardo Vasta and Adam G. Marsh. Center of Marine Biotechnology. University of Maryland Biotechnology Institute.
The Role of Iron and Host-Derived Growth-Factors in Regulating Gene Expression in the Oyster Parasite Perkinsus marinus: Strategies for Inhibiting Proliferation. 1996-1997. Gerardo Vasta and Adam G. Marsh. Center of Marine Biotechnology. University of Maryland Biotechnology Institute.
The Molecular Basis for the Etiology of the Oyster Dermo Disease: Gene Regulation Events Susceptible to Chemical Inhibition. 1996-1997. Gerardo Vasta and Adam G. Marsh. Center of Marine Biotechnology. University of Maryland Biotechnology Institute.
A Molecular Approach to Environmental Studies on Perkinsus marinus. Transmission Dynamics of Infection in Chesapeake Bay. 1999-1999. Gerardo Vasta. Center of Marine Biotechnology. University of Maryland Biotechnology Institute.
**Use of images with permission, J. Adam Frederick**





