Knauss legislative fellowships in Congress help build careers — and they're fun and educational. See our video and fact sheet for details.
Will Ocean Acidification Create “Super Crabs” in Bay? Maybe Not
Acidifying Conditions Could Bring Benefits and Problems for the Bay's Iconic Crustaceans
Sorry to disappoint comic-book fans, but don’t expect to see any crabs with super powers swimming around the Chesapeake Bay — despite the Washington Post's prediction not long ago that "it is the dawn of the super crab."
The statement might conjure images of caped crustaceans battling super villains beneath the waves of the Chesapeake. But recent research findings suggest the real story packs less comic-book zap -- but nevertheless some intriguing insights about shellfish biology.
The “super crab” alarm was tied to an important research question: what will happen to blue crabs as the Bay becomes more acidic? Scientists say that acidity levels in the estuary, and in water bodies across the world, will climb in the decades ahead — a trend that’s called ocean acidification. It’s caused mostly by rising concentrations of carbon dioxide in the atmosphere; some of the CO2 becomes dissolved in the ocean.
The trend is undoubtedly bad for some Bay organisms, such as oysters and clams, which struggle to build their rock-like shells in more acidic environments. However, a 2009 study indicated that blue crabs might benefit from ocean acidification, growing shells in the future that are bulkier, not wimpier. What’s more, blue crabs like to prey on oysters — hence the concern about super-shelled critters.
But like most things in the Bay, the reality is more complicated, says Hillary Glandon. She’s a graduate student at the Chesapeake Biological Laboratory of the University of Maryland Center for Environmental Science (UMCES). It’s her job to dig into the fate of blue crabs affected by ocean acidification.
Previous research findings surrounding acidification could be misinterpreted to suggest that “we’re going to have a Bay full of crabs and no oysters,” says Lane, who studies with UMCES fisheries scientist Thomas Miller. But, Glandon says, crabs don’t live in a vacuum.
To begin, it’s important to understand some details about shellfish chemistry, details that help to explain why a crab’s exoskeleton is different from an oyster’s shell. Both oyster shells and crab exoskeletons are rich in calcium carbonate, the same chalk-like material that gives corals their toughness. The two Bay animals, however, make this important mineral in different ways. Oysters use a molecule called carbonate — a carbon atom bonded to three oxygen atoms — as the main building block in their shells, grabbing it from the water around them. Crabs, on the other hand, use bicarbonate, which has an extra hydrogen atom. Think of it like two masons, one of whom prefers to build with granite and the other with limestone.
In water bodies like the Bay, ocean acidification can alter the concentration of these building blocks and their availability to these animals. As the water becomes more acidic, fewer bicarbonate ions are transformed chemically into carbonate ions. Or as Glandon puts it, “the form of carbonate that crabs use becomes more available, and the form that oysters and clams use becomes less available.”
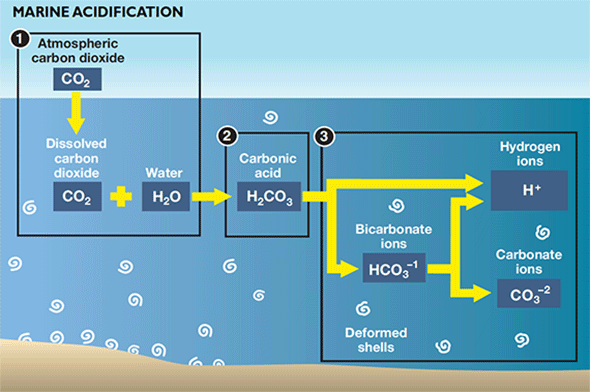 |
| Carbonic acid in ocean and Bay waters breaks down into bicarbonate and hydrogen ions (H+). Bicarbonate breaks down into more H+ and carbonate, which helps organisms like oysters, clams, corals, and other marine organisms form shells and skeletons. But as acidity increases, less bicarbonate changes into carbonate. Credit: Sandy Rodgers, Chesapeake Quarterly |
It may make a difference: A laboratory study published in 2009 by researchers at the University of North Carolina found that crabs raised in acidic waters tended to grow thicker shells than their counterparts in normal ocean water. But was it a simple case of crabs becoming super, while oysters became weaklings?
That’s what Glandon wanted to find out. In summer 2014, she conducted a study, rearing juvenile blue crabs in normal estuary water and also in acidic surroundings. She mixed high levels of carbon dioxide gas into some of the crabs’ water, simulating conditions that the animals might face in the future if human greenhouse gas emissions continue to spike.
Glandon was less interested in the crabs’ shells and more focused on the growth and survival of the youngsters. And her preliminary results suggest that crabs raised under more acidic conditions grew more slowly than crustaceans swimming in 2014 conditions. In other words, the crabs living in acidic waters struggled to pack on weight as fast as their less acidic counterparts did. Growing fast is an important task for an animal that is food for bigger fish predators until it reaches a certain size.
The reason for the difference in growth rates isn’t clear, Glandon says. She suspects that young crabs, like most animals, have evolved to live under a certain range of environmental conditions. Change those conditions by too much, by altering the acidity levels in their water, and the animals’ growth could get thrown off.
So don’t expect to see the Bay become overrun by “super crabs” anytime soon, she says. What exactly will happen to crabs in an acidifying environment isn’t clear, the graduate student notes. But Glandon plans to continue to delve into that dilemma. Her next set of experiments will explore another side effect of rising carbon dioxide in the atmosphere — warming water temperatures — and how these might also affect the growth of young crabs. She says that the question is an important one because it has implications not only for the survival of crabs in the Bay, but also for the livelihood of the watermen that harvest these animals.
Photo, top left: Crabs grown experimentally at high levels of carbon dioxide (10 times pre-industrial levels) developed larger shells than those grown at today's levels, a 2009 study found. The scale is in centimeters after 60 days of growth. Credit: Justin Ries, University of North Carolina at Chapel Hill
For further reading, see an article from Maryland Sea Grant's magazine, Chesapeake Quarterly, "Crab vs. Oyster: As Acidity Increases, Some Species May Win and Others Lose."
See all posts from the On the Bay blog







